Centralized Catalog, Faster Publishing: 40ParkLane’s Marketplace Success with CedCommerce
Reading Time: 4 minutesAbout the Brand: 40ParkLane LLC Studio40ParkLane is a design-led print-on-demand brand created…
TikTok Shop has released a comprehensive Beauty and Personal Care Products Policy, outlining the documentation, qualification, and regulatory requirements sellers must meet to list products in this category. The policy reflects TikTok Shop’s broader push toward stronger consumer protection, regulatory alignment, and platform trust—particularly for products applied to the skin, hair, or body.
The updated framework applies to manufacturers, importers, repackers, and resellers, with requirements varying based on the seller’s role and the nature of the product being listed.
TikTok Shop defines beauty and personal care products as items designed for cleansing, beautifying, and supporting hygiene and personal well-being. These products span all age groups, including babies, children, and adults.
Covered product types include, but are not limited to:
Some products in this category are also regulated as over-the-counter (OTC) drugs, meaning they provide both cosmetic and health-related benefits. Examples include:
These dual-use products are subject to additional labeling and documentation standards.
To sell beauty and personal care products, sellers may be required to complete a category qualification process through TikTok Shop’s Qualification Center. This process verifies that sellers meet regulatory and safety standards before their products are approved for sale.
TikTok Shop advises sellers to review the qualification process carefully and consult its category qualification guidance, particularly when handling rejections or resubmissions.
Sellers who manufacture, import, or repack beauty and personal care products may be required to submit multiple forms of documentation.
Eligible sellers must provide proof of active FDA registration, including:
Acceptable proof includes:
Sellers qualifying for a MoCRA exemption, such as certain small businesses, must submit a self-attested document confirming their exemption eligibility.
Cosmetic labels must include:
If the product provides medical or health benefits, a Drug Facts label is required, including:
Resellers are subject to a separate documentation pathway.
Resellers may need to submit a valid purchase invoice that:
Resellers must also provide cosmetic labels and, where applicable, Drug Facts labels that meet the same standards required of manufacturers.
TikTok Shop explicitly prohibits the sale of several types of beauty and personal care products, including:
Beyond TikTok Shop’s internal policies, sellers are responsible for complying with all applicable laws, including:
A significant compliance focus is the Modernization of Cosmetics Regulation Act of 2022 (MoCRA), which introduces new federal obligations for cosmetic products sold in the U.S.
Key MoCRA requirements include:
TikTok Shop conducts regular compliance reviews. Violations may result in actions such as:
Sellers may appeal enforcement decisions through TikTok Shop’s official appeals process.
Sellers must submit all required documents via the Qualification Center in Seller Center by:
Most rejections occur due to missing or incomplete documentation. Sellers can review rejection reasons directly in Seller Center notifications or within the Qualification Center.
To reapply, sellers must:

Reading Time: 4 minutesAbout the Brand: 40ParkLane LLC Studio40ParkLane is a design-led print-on-demand brand created…
Reading Time: 3 minutesAbout the Company Brand Name: David Protein Industry: Health & Nutrition (Protein…

Reading Time: 3 minutesOnline retail spending in Germany is entering a renewed growth phase after…

Reading Time: 4 minutesTikTok Shop has released a comprehensive Beauty and Personal Care Products Policy,…

Reading Time: 4 minutesTikTok Shop has formally outlined comprehensive requirements for expiration date labeling and…

Reading Time: 3 minutesTikTok Shop is raising its sales commission for merchants across five active…

Reading Time: 11 minutesBy now you have seen your BFCM 2025 numbers. The harder question…
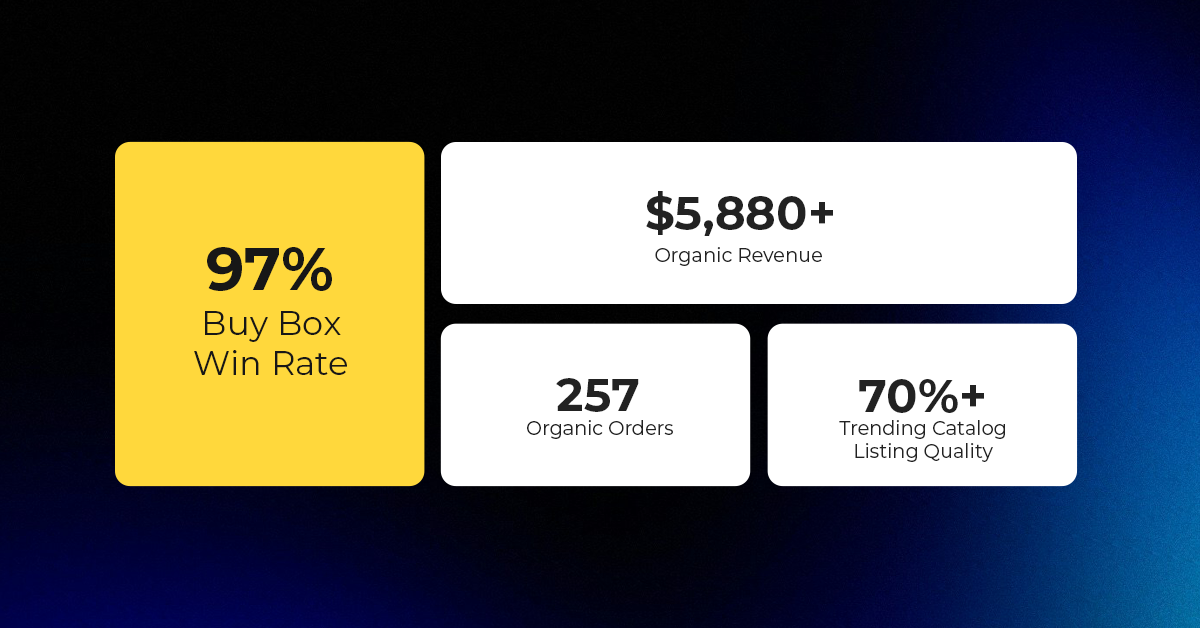
Reading Time: 3 minutesAbout the Brand Name: Vanity Slabs Inc Industry: Trading Slabs- Vanity Slabs…
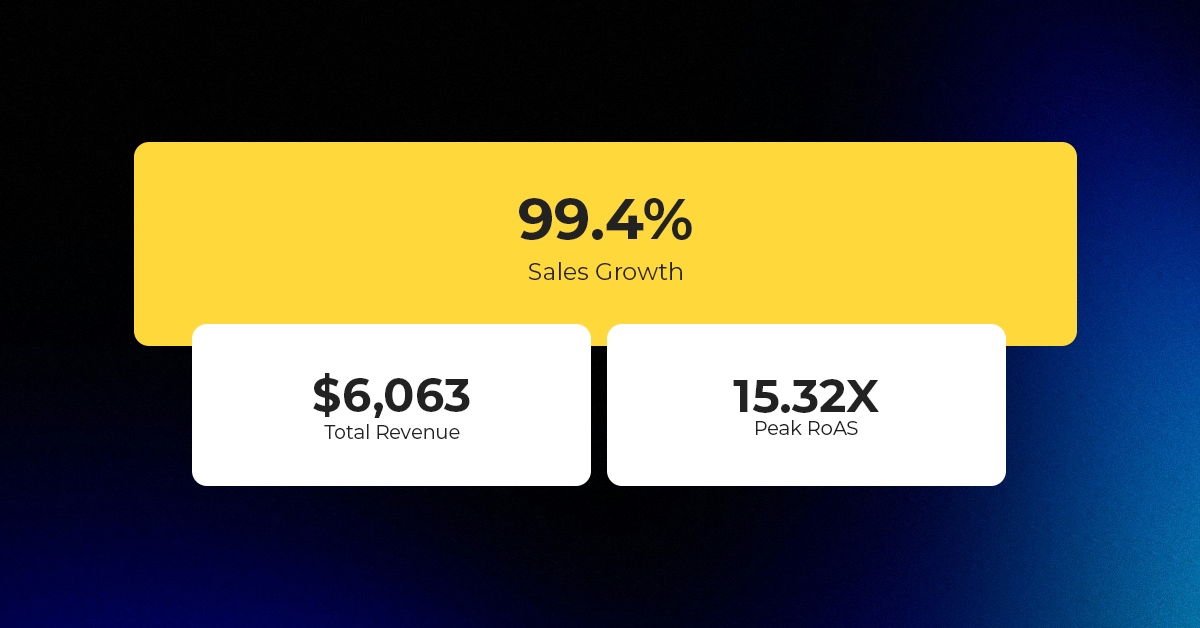
Reading Time: 2 minutesAbout the Brand Name: Ramjet.com Industry: Automotive Parts & Accessories Location: United…

Reading Time: 2 minutesAmazon is rolling out strategic referral fee reductions across five major European…

Reading Time: 4 minutesQuick Summary: Scaling Lifestyle Powersports on eBay with CedCommerce Challenge: Zero marketplace…

Reading Time: 4 minutesTikTok has surpassed 460 million users across Southeast Asia, reinforcing its position…

Reading Time: 3 minuteseBay has released its final seller news update for 2025, with a…

Reading Time: 3 minutesAmazon has clarified its stance regarding speculation around a potential breakup between…

Reading Time: 4 minutesWalmart is accelerating its push into next-generation fulfillment by expanding its drone…

Reading Time: 4 minutesFaire, the fast-growing wholesale marketplace connecting independent retailers with emerging brands, has…

Reading Time: 4 minutesB2B buying in the United States is undergoing a fundamental behavioral shift…

Reading Time: 3 minutesSummary Cyber Monday 2025 has officially become the largest online shopping day…

Reading Time: 2 minutesSummary Amazon kicked off December with two major developments shaping the future…

Reading Time: 2 minutesSummary Walmart has entered December with two major moves that signal a…